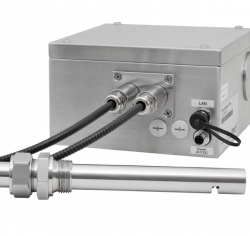
NIR (Near Infrared) Spectrometer and Analyzer
Description
The tec5USA NIR spectrometer system is available in wavelength ranges spanning 960 – 2150 nm with a wavelength accuracy of ± 1 nm and spectral resolution of ∆λFWHM 5 – 16 nm. The spectrometer comes with a fully process capable communications system that can be interfaced with distributed control systems for online process control and automation. The InGaAs Photodiode Array detector, available in either 256 or 512 pixel versions, utilizes holographic gratings which have been chosen to give both good flat field correction of the spectral imaging and maximum efficiency in the near-infrared wavelength range. The device is equipped with multiplexing operation capability, allowing for a connection to up to 8 measurement locations with a single device. The spectrometer is designed to be rugged and environmentally insensitive, so measurements are minimally affected by environmental changes, allowing for high accuracy and high precision measurements for real-time process monitoring and control.
Features
- Extended InGaAs technology
- 960 - 2170 nm available spectral range
- Long-life halogen light source
- Optimized gratings for NIR wavelength range
- Fast, precise & robust
Advantages
- Multichannel operation capability
- Real-time quantitative and qualitative results
- Multi-component analysis
- High reproducibility and long-term stability
- Modular concept with high flexibility
Specifications
- Light source Long-life halogen, 20 - 50 W
- Fiber Coupling SMA
- Detector Type Extended, thermoelectrically cooled InGaAs
- Wavelength Accuracy 0.6 nm
- Wavelength Range/Resolution 960 – 1690 nm (PGS 1.7) / 5 nm 1340 - 2000 nm (PGS 2.0) / 6 nm 1000 - 2150 (S2.0) / 16 nm
- Integration time 0.1 ms - 1.5 s
- Readout time 3.4 ms
- PC Interfaces Ehternet, Highspeed USB
- Number of Channels Up to 8
see also those other